Covid Vaccination Pregnancy Europe
Pregnant women with coronavirus disease 2019 Covid-19 are at increased risk for adverse outcomes and Covid-19 vaccination is recommended during pregnancy. USA Canada and Europe.
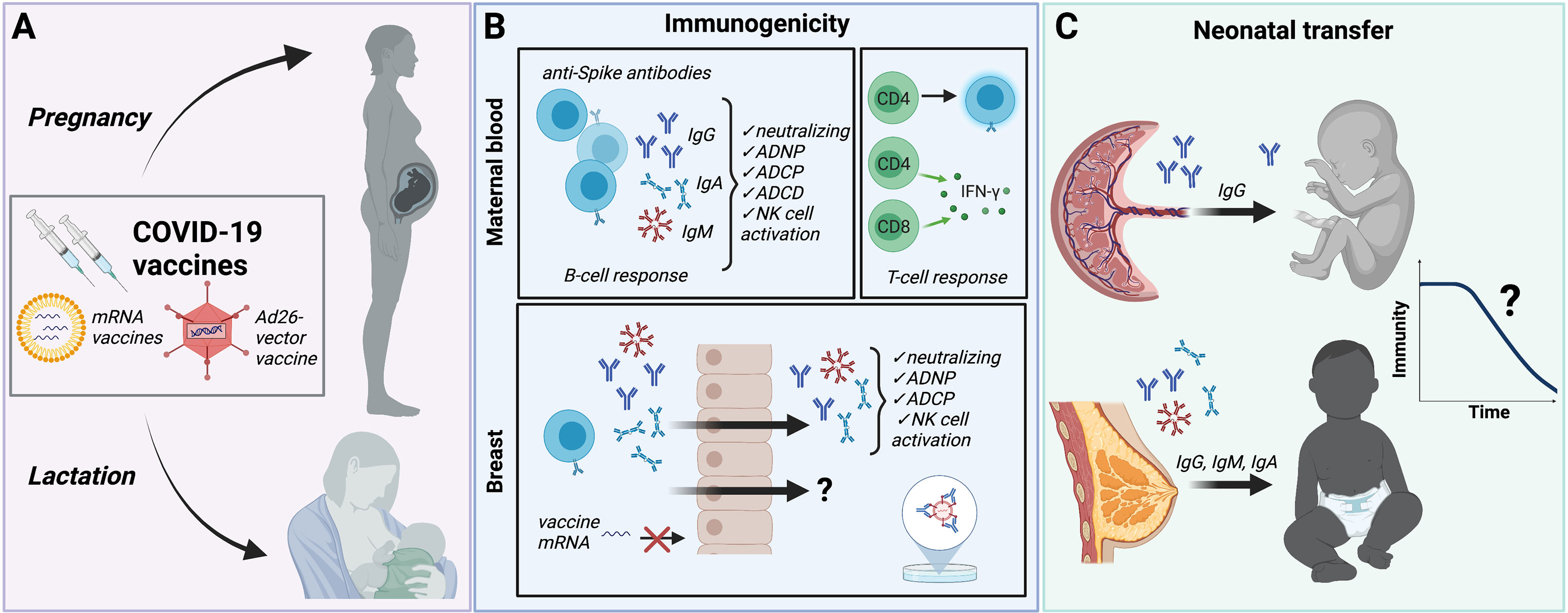
Frontiers Covid 19 Vaccination In Pregnancy And Lactation Current Research And Gaps In Understanding Cellular And Infection Microbiology
EMA is not involved in advising on travel requirements in the European Union EU such as vaccination quarantine or testing for travellers.
Covid vaccination pregnancy europe
. COVID-19 vaccine in pregnancy. Look up product expiration dates using your smartphone. Although the benefitrisk profile of these vaccines. Real-world data from the United States has shown that around 90000 pregnant.Vaccination is the best way to protect the mother and fetus against the known risks of COVID-19 in pregnancy. Up to 12 cash back To date four vaccines have been authorised for emergency use and under conditional approval by the European Medicines Agency to prevent COVID-19. Comirnaty COVID-19 Vaccine Janssen Spikevax previously COVID-19 Vaccine Moderna and Vaxzevria previously COVID-19 Vaccine AstraZeneca. The US Centers for Disease Control and Prevention CDC and the European Medicines Agency EMA say COVID-19 vaccines.
CDC V-Safe COVID-19 Vaccine Pregnancy Registry. Look up product expiration dates using your smartphone. Decisions about which COVID-19 vaccines are included for example in the EU Digital COVID Certificate are taken by the EU Member StatesEMA is in charge of the scientific evaluation of vaccines. In the USA over 100000 pregnant women have been vaccinated mainly with Pfizer and Moderna vaccines and no safety concerns have been.
Ad See required Emergency Use Authorization EUA and safety information. To date four vaccines have been authorised for emergency use and under conditional approval by the European Medicines Agency to prevent COVID-19. The COVID-19 m-RNA vaccines Comirnaty developed by PfizerBiontech and Spikevax previously COVID-19 Vaccine Moderna and the COVID-19 viral vector vaccines COVID-19 Vaccine Janssen and Vaxzevria previously COVID-19 Vaccine. In the USA Europe and globally health authorities have published various COVID-19 vaccine recommendations for women who may become pregnant pregnant women and women breastfeeding their child.
Including pregnant women can also be referred through that system to a vaccination site at a hospital in central. Ad See required Emergency Use Authorization EUA and safety information. COVID-19 vaccination in pregnancy. The Joint Committee on Vaccination and Immunisation JCVI has advised that pregnant women should be offered COVID-19 vaccines at the same time as people of the.
While pregnant and lactating women were largely not included in COVID-19 vaccine trials two studies yesterday in Science Translational Medicine look at how the mRNA COVID-19 vaccines. Online ahead of print. In conclusion the COVID-19 vaccines are safe effective and highly recommended in pregnancy. But there is confusion about the facts.
In view of this data vaccination can be considered for pregnant women from the 2nd trimester period carrying a lower risk of teratogenic effects and pregnancy termination particularly in the presence of important risk factors obesity diabetes etc for severe COVID. Pregnant women are vulnerable to COVID-19 with increased risk of more severe illness and pregnancy complications particularly if infected during the third trimester1 Based on prior experience with vaccines in pregnancy and with no hypothesised mechanisms for fetal harm similar efficacy and side-effects to the non-pregnant population were anticipated with vaccination. The JCVI has advised that it is now preferable for pregnant women in the UK to be offered the Pfizer-BioNTech or Moderna vaccines. Comirnaty COVID-19 Vaccine Janssen Spikevax previously COVID-19 Vaccine Moderna and Vaxzevria previously COVID-19 Vaccine.
The decision to offer pregnant women the Covid-19 vaccines is based on real-world data from the US where 130000 pregnant women have been vaccinated mainly with mRNA vaccines including Pfizer-BioNTech or Moderna vaccines. Numerous countries have this year recommended that pregnant have COVID-19 vaccinations after finding them to be safe. COVID-19 vaccination in pregnancy. This information helps CDC monitor the safety of COVID-19 vaccines in people who are pregnant.
On this page we outline key findings of a global survey conducted between Oct. The Joint Committee on Vaccination and Immunisation JCVI has issued new advice for pregnant women on taking the COVID-19 vaccine. A registry to collect additional health information from v-safe participants who report being pregnant at the time of vaccination or a positive pregnancy test after vaccination. Members of the JCVI said only about 15 of pregnant women in the UK have been fully vaccinated.
Gynecologists in Germany want pregnant women to be prioritized for COVID-19 vaccinations. 18 2020 which assessed the acceptance of COVID-19 vaccination including potential predictors among pregnant. Covid Vaccine Effort in Europe Confronts Anger Disinformation and Suspicion. A Covid vaccination sign on the A4 in Buckinghamshire.
The European Medicines Agency said in July that data seen. 12 However safety data on Covid. To date four COVID-19 vaccines have been authorised for emergency use in Europe starting from December 2020.
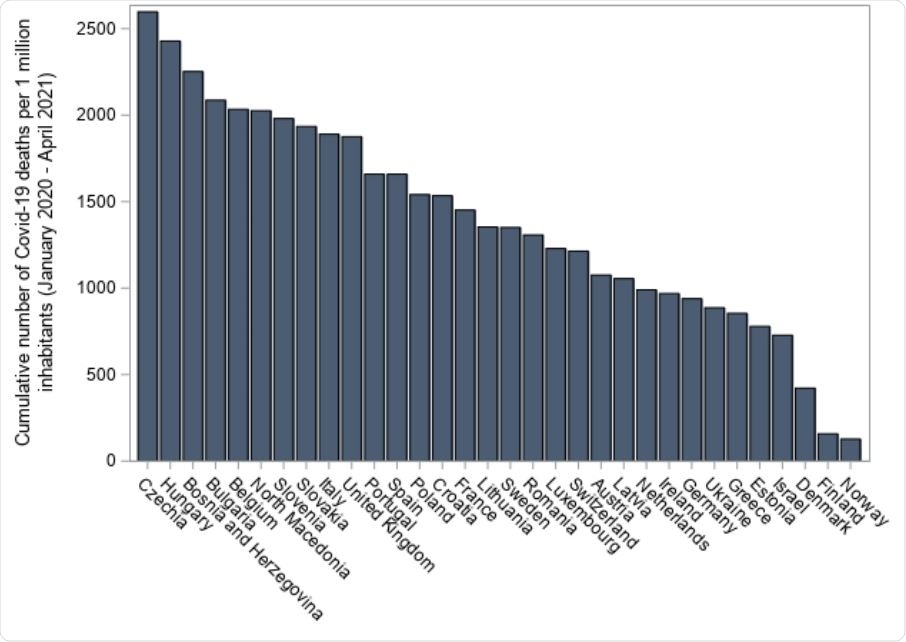
Covid 19 Mortality In Israel And Europe After Vaccination

Covid 19 Vaccine Acceptance Among Pregnant Women And Mothers Of Young Children Human Immunomics Initiative

Covid 19 Vaccination Advice Upgraded Yet Again For Those Pregnant Or Planning Pregnancy

Excellence In Pediatrics Together Platform

Covid 19 Vaccine Acceptance Among Pregnant Women And Mothers Of Young Children Human Immunomics Initiative
Posting Komentar untuk "Covid Vaccination Pregnancy Europe"