why are valence electrons important
See full answer below. Similarly what does the electron configuration tell you.

Valence Electron And Electric Conductivity Electrical4u
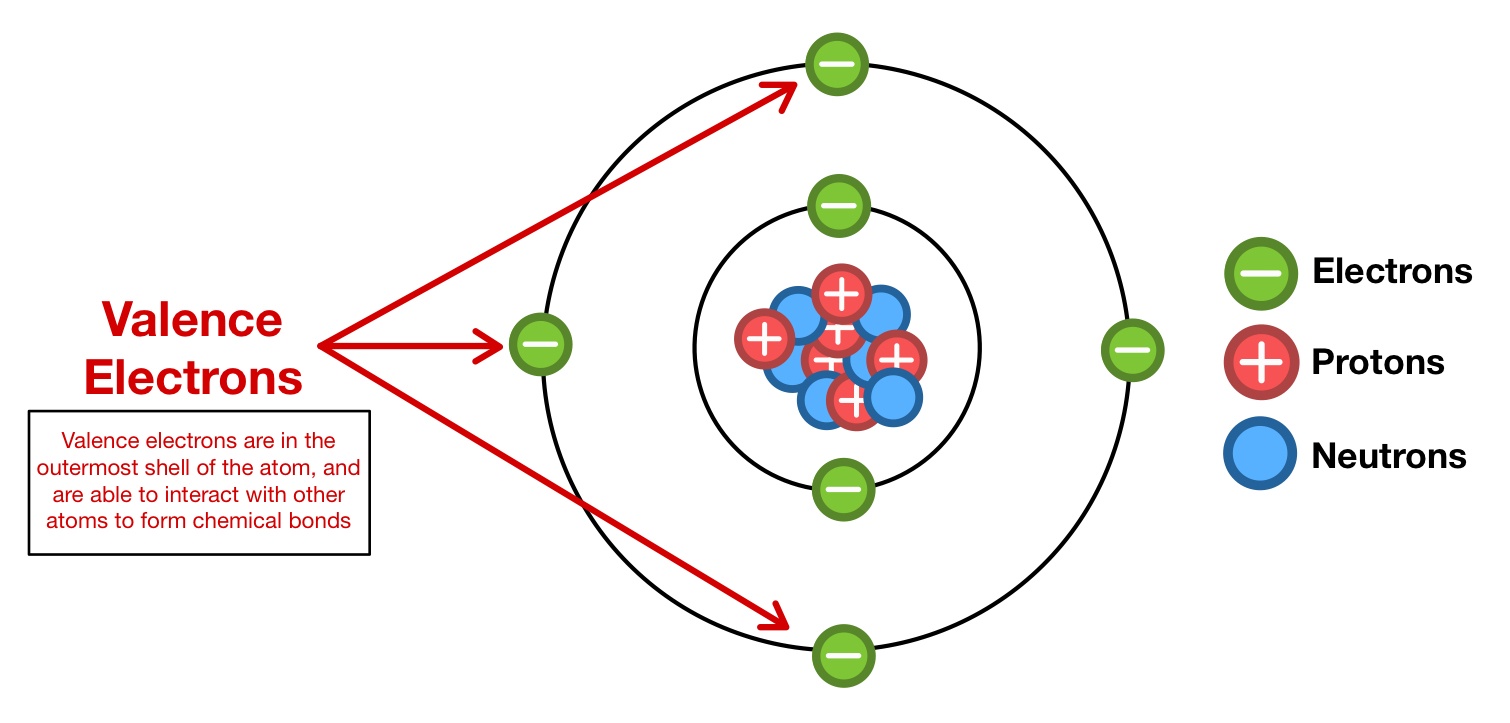
The electrons that occupy the outermost shell of an atom are called valence electrons.

. Why is the electron so important. The valence electrons refer to the electrons present in the outer shell of an element. Since filled d or f subshells are seldom disturbed in a chemical reaction we can define valence electrons as follows. Valence electrons are important because they determine how an atom will react.
Importance of Valence Electrons. Click to see complete answer. Because valence electrons are by definition the outermost part of the atom. Why are valence electrons important in chemical reactions.
This means that all of the molecules which govern life-giving chemistry have carbon atoms as their principal component. The student will be able to write the electron configuration of any element given the atomic number. The electrons that occupy the outer most shell of an atom are called valence electrons. So on that basis elements are.
Why are valence electrons important. They are important to an atom because the fewer valence electrons that the atom holds the less stable it becomes. This is important because valence electrons contribute to the unique chemistry of each atom. The bonding between the two atoms is due to the interaction of those.
If the valence electrons of elements are really close or really far to 8 like 1 or 7 those elements tend to be very reactive and dont generally have a lot of oxidation states. Note that the number of valence electrons. Electrons orbit the nucleus of an atom in various energy levels. They are important to an atom because the fewer valence electrons that the atom holds the less stable it becomes.
Are the outer electrons and therefore they are the first ones to interact when two atoms come near each other. Correspondingly what is special about valence electrons. They are important because they affect how an atom responds since the number of electrons that share the maximum energy level by composing an electron configuration can be seen. Thornwood High School 339-7800 x 2785 Objectives.
It is the outer energy level that wants to gain or lose electrons to make a complete or full energy level. Based on the valence electrons the formation of ionic and covalent bonds takes place. The electrons in the outermost shell are the valence electrons the electrons on an atom that can be gained or lost in a chemical reaction. They are important because they determine how an atom will react.
Thus they are the parts of the atom that interact with other atoms just as the skin of an orange is the part that interacts with the outside world. To predict molecular shape we usually use a theory called the valence shell electron pair VSEPR repulsion theoryThis theory is based on the idea that valence electrons in a molecule. Create your account View this answer Valence electrons. They are important in chemistry because they are responsible for determining the chemical behavior of an atom.
They are important because they determine how an atom will react. The nature of the interaction between the atoms depends on their relative electronegativity. Similarly it is asked why are valence electrons important for determining molecular shape. By writing an electron configuration Youll be able to see how many electrons occupy the highest energy level.
Given the location of an element on the periodic table the student will be able to give the highest energy level the valence electrons are on and how many valence electrons it. I also believe that the number of valence electrons of an element determine what group on the periodic table of elements it is in. These are the electrons that possess the tendency to either share with other elements to give rise to molecules or to lose completely to turn into an ion. Click to see full answer.
Tend to repel each other to create more space around them. It tells you how many electrons there are in each of the different orbitals s p d or f and at which energy level n 1 2 3. The alkali metals group 1 elements each have 1 valence electrons so they tend to be very reactive and readily lose that electron. Valence electrons are important as they indicate an elements bonding behavior stability and reactivity.
Sikringbp and 7 more users found this answer helpful. By writing an electron configuration youll be able to see. Why is there no hydrogen bond in HCl. When two atoms approach each other and react with each other it is their outer shells that come into contact first and it is therefore the electrons in their outer shells that are normally involved in any chemical reaction.
What is valence electrons in chemistry. To form an inert gas electron configuration each atom in HCl requires one more electron. Valence electrons are important because they are the electrons that each atom uses to bond or that can be stripped from the atom to create an ion. The electrons on an atom that are not present in the previous rare gas ignoring filled d or f subshells.
Valence electrons are the electrons that reside in the outermost electron shell of an atom in the highest energy level. Why Are Electrons Important. Why are electrons so important. Valence electrons are those electrons farthest away from the nucleus of an atom.
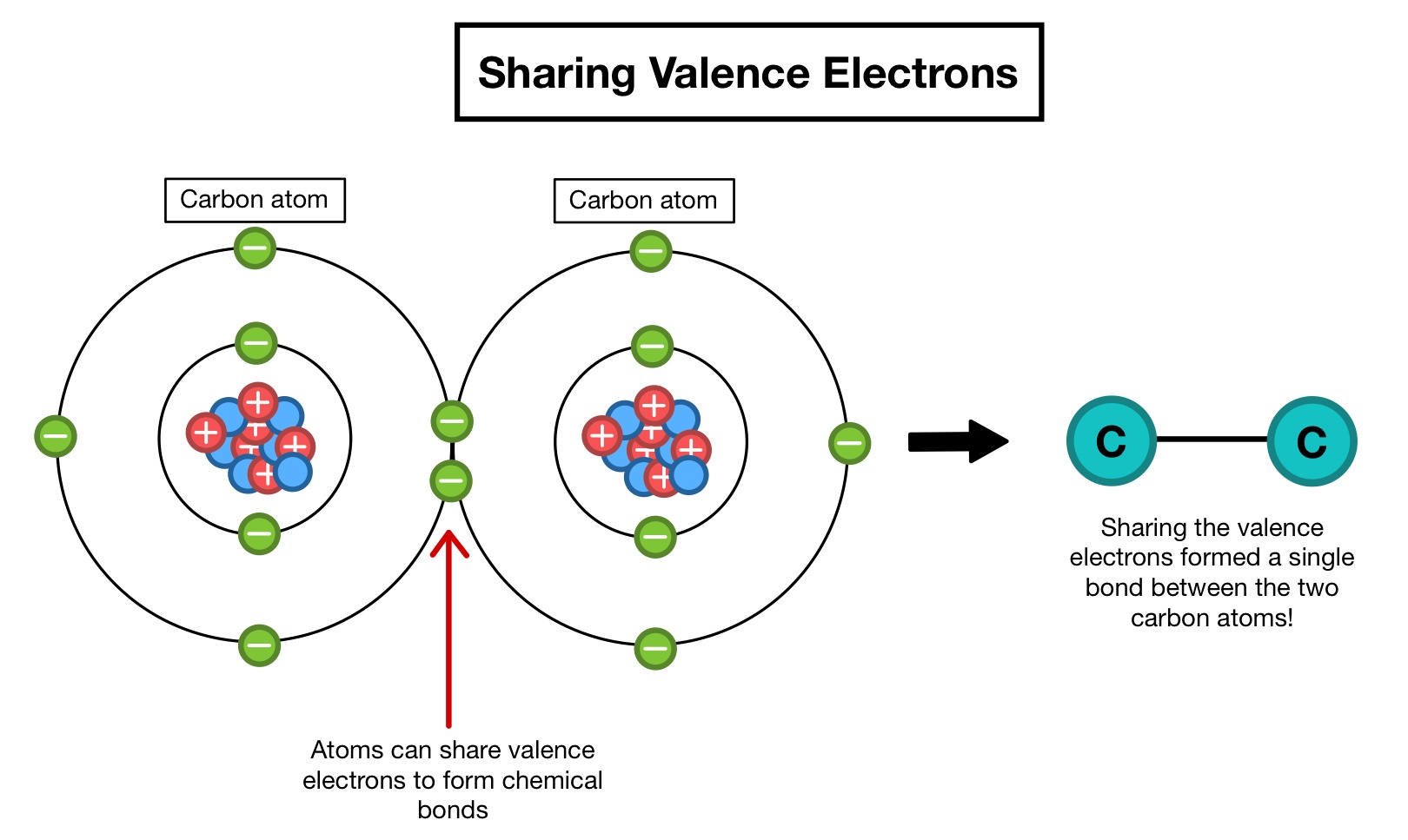
Non-valence electrons are not easily removed from. The number of valence electrons the number of electrons in the outer orbital lets the atom combine with other elements that want more valence electrons. Bonds are formed when valence electrons the electrons in the outermost electronic shell of an atom interact. It is the valence electrons that have a reaction with other atoms.
Valence electrons are the electrons that occupy an atoms outermost shell. By writing an electron configuration Youll be able to see how many electrons occupy the highest energy level. The electrons that occupy the outer most shell of an atom are called valence electrons. Valence electrons are the electrons that reside in the outermost electron shell of an atom in the highest energy level.
Valence Electrons Definition Importance Expii

How To Find Valence Electrons Chemical Equation Electron Configuration Electrons


Posting Komentar untuk "why are valence electrons important"